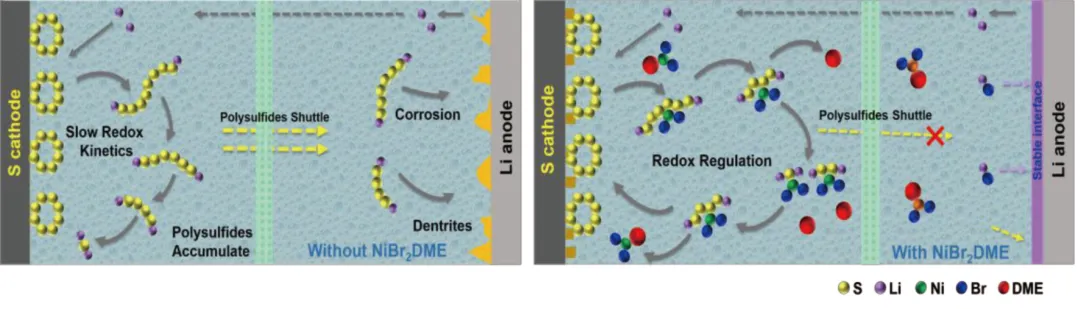
锂硫电池具有较高的理论比能,被认为是极具发展前景的下一代储能系统之一。然而,多硫化锂的穿梭效应和锂阳极的界面不稳定性严重阻碍了锂硫电池的实际应用。利用添加剂优化电解液的组成可以显著提高电池的性能。
近日,南昌大学杨震宇教授和张泽博士等人在Science China Materials发表研究论文,提出了一种有机金属盐,即溴化镍-二甲氧基乙烷(NiBr2DME)作为电解质添加剂,它具有调节LiPSs氧化还原和稳定锂阳极的双重功能。
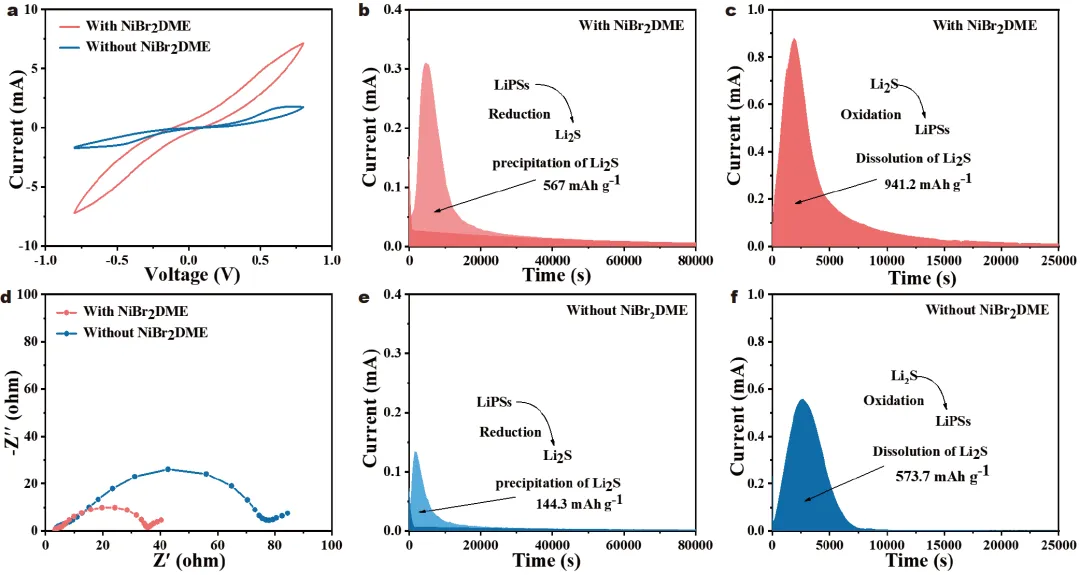
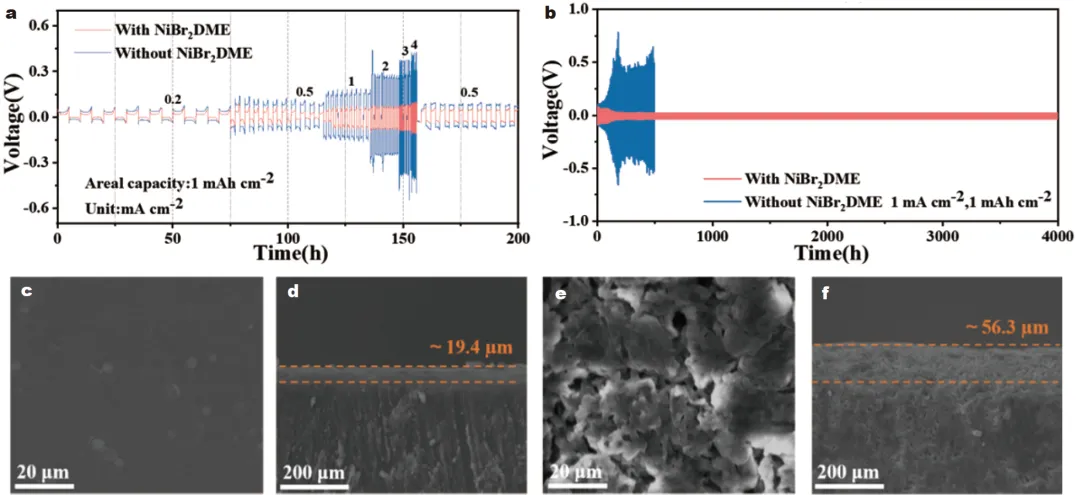
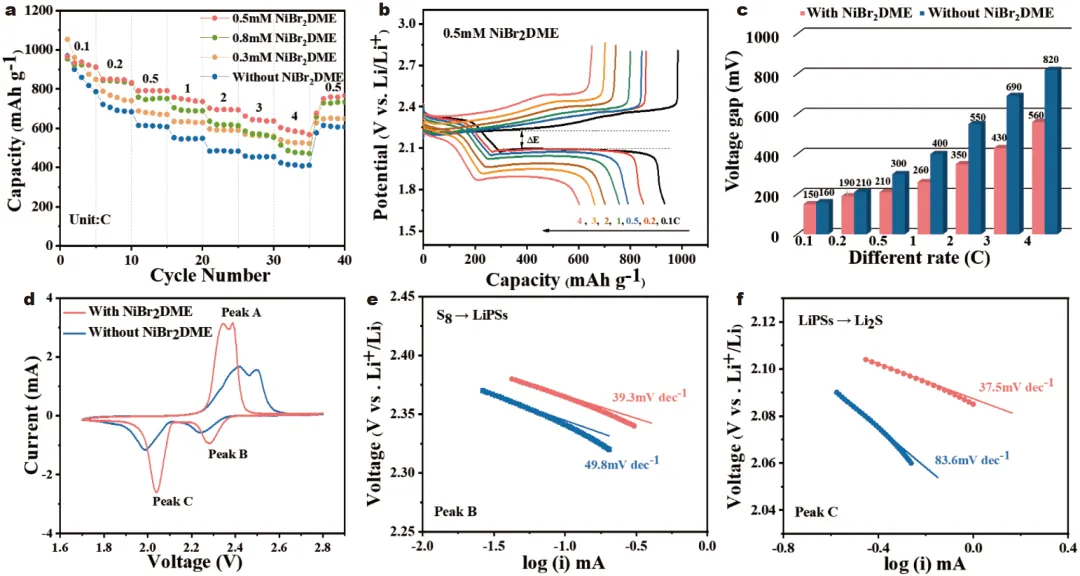
1) 发现NiBr2DME可以通过Ni–S和Li–Br键与LiPSs相互作用,加速了LiPSs的相互转化,从而减少了LiPSs在电解质中的积累。此外,NiBr2DME能在锂金属表面形成稳定的含LiBr界面层,促进锂离子的均匀电沉积,抑制锂枝晶的形成。2) 使用浓度为0.5 mmol L−1 NiBr2DME的Li-S电池在0.2 C下的初始容量为919.8 mAh g−1,循环100次后容量保持率高达89.3%。即使在4 C的速率下,也可以实现602.9 mAh g−1的高放电容量。在含硫量为4.8 mg cm−2、液硫比为5 μL mg−1的较差电解质条件下,电池仍能保持良好的循环性能。本研究为实现抑制穿梭效应、调控LiPSs氧化还原和稳定锂阳极提供了积极的解决方案。Figure 1. Schematic diagram of working principle of Li-S batteries with and without NiBr2DME additive.Figure 2. (a) CV curves, and (b) EIS plots of Li2S6 symmetric cells. (c, d) Static discharge curve of Li2S deposition, and (e, f) Dissolution curve of Li2S with/without the NiBr2DME additive.Figure 3. Electrochemical performance of Li/Li cells with/without NiBr2DME additive. (a) Rate performance at different current densities with a fixed area capacity of 1 mAh cm−2. (b) Cyclic stability at 1 mA cm−2. SEM images of cycled Li metal (c, d) with and (e, f) without NiBr2DME additive.Figure 4. Electrochemical performance of Li-S batteries with/without NiBr2DME. (a) Rate performance. (b) Constant-current charge-discharge curves. (c) Polarization potential at different current densities. (d) CV curves of Li-S cells with/without the NiBr2DME. Tafel plots of (e) Peak B, and (f) Peak C.Yixuan Meng, Meifang Zhang, Youliang Wang, Chen Liu, Ze Zhang, Ji Yu, Jianxin Cai, Zhenyu Yang. An organometallic salt as the electrolyte additive to regulate lithium polysulfide redox and stabilize lithium anodes for robust lithium-sulfur batteries. Sci. China Mater. (2024).https://doi.org/10.1007/s40843-024-2969-3
来源:中国科学材料